Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yotsuji, Kenji*; Tachi, Yukio; Sakuma, Hiroshi*; Kawamura, Katsuyuki*
Applied Clay Science, 204, p.106034_1 - 106034_13, 2021/04
Times Cited Count:61 Percentile:99.73(Chemistry, Physical)Gonzal z, M. A.*; Borodin, O.*; Kofu, Maiko; Shibata, Kaoru; Yamada, Takeshi*; Yamamuro, Osamu*; Xu, K.*; Price, D. L.*; Saboungi, M.-L.*
z, M. A.*; Borodin, O.*; Kofu, Maiko; Shibata, Kaoru; Yamada, Takeshi*; Yamamuro, Osamu*; Xu, K.*; Price, D. L.*; Saboungi, M.-L.*
Journal of Physical Chemistry Letters (Internet), 11(17), p.7279 - 7284, 2020/09
Times Cited Count:16 Percentile:77.33(Chemistry, Physical)Yotsuji, Kenji*; Tachi, Yukio; Kawamura, Katsuyuki*; Arima, Tatsumi*; Sakuma, Hiroshi*
Nendo Kagaku, 58(1), p.8 - 25, 2019/00
Molecular dynamics (MD) simulations were conducted to investigate physical properties of water and cations in montmorillonite interlayer nanopores. The swelling behaviors and hydration states were firstly evaluated as functions of interlayer cations and layer charge. The diffusion coefficients of water and cations in interlayer nanopores were decreased in comparison with those in bulk water and came closer to those in bulk water when basal spacing increased. The viscosity coefficients of interlayer water estimated indicated a significant effect of viscoelectricity at 1- and 2-layer hydration states and higher layer charge of montmorillonite. These trends from MD calculations were confirmed to be consistent with existing measured data and previous MD simulation. In addition, model and parameter related to viscoelectric effect used in the diffusion model was refined based on comparative discussion between MD simulations and measurements. The series of MD calculations could provide atomic level understanding for the developments and improvements of the diffusion model for compacted montmorillonite.
Arima, Tatsumi*; Idemitsu, Kazuya*; Inagaki, Yaohiro*; Kawamura, Katsuyuki*; Tachi, Yukio; Yotsuji, Kenji
Progress in Nuclear Energy, 92, p.286 - 297, 2016/09
Times Cited Count:11 Percentile:68.36(Nuclear Science & Technology)Diffusion and adsorption behavior of uranyl (UO ) species is important for the performance assessment of radioactive waste disposal. The diffusion behaviors of UO
) species is important for the performance assessment of radioactive waste disposal. The diffusion behaviors of UO , K
, K , CO
, CO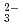 and Cl
and Cl and H
and H O in the aqueous solutions were evaluated by molecular dynamics (MD) calculations. The diffusion coefficient (De) of UO
O in the aqueous solutions were evaluated by molecular dynamics (MD) calculations. The diffusion coefficient (De) of UO is the smallest and is 26% less than the self-diffusion coefficient of H
is the smallest and is 26% less than the self-diffusion coefficient of H O. For the aqueous solution with high concentration of carbonate ions, uranyl carbonate complexes: UO
O. For the aqueous solution with high concentration of carbonate ions, uranyl carbonate complexes: UO CO
CO and UO
and UO (CO
(CO )
) can be observed. For the clay (montmorillonite or illite)-aqueous solution systems, the adsorption and diffusion behaviors of UO
can be observed. For the clay (montmorillonite or illite)-aqueous solution systems, the adsorption and diffusion behaviors of UO and K
and K were evaluated by MD calculations. The distribution coefficients (Kd) increase with the layer charge of clay, and Kd of UO
were evaluated by MD calculations. The distribution coefficients (Kd) increase with the layer charge of clay, and Kd of UO might be smaller than that of K
might be smaller than that of K . Further, their two-dimensional diffusion coefficients were relatively small in the adsorption layer and were extremely small for illite with higher layer charge.
. Further, their two-dimensional diffusion coefficients were relatively small in the adsorption layer and were extremely small for illite with higher layer charge.
Yonetani, Yoshiteru
Chemical Physics Letters, 406(1-3), p.49 - 53, 2005/04
Times Cited Count:52 Percentile:84.75(Chemistry, Physical)We report that a severe artifact appeared in molecular dynamics simulation of bulk water using the long cut-off length 18 AA ; Our result shows that increasing the cut-off length does not always improve the simulation result. Moreover, the use of the long cut-off length can lead to a spurious result. It is suggested that the simulation of solvated biomolecules using such a long cut-off length, which has been often performed, may contain an unexpected artifact.
*; Kunugi, Tomoaki
Journal of Nuclear Materials, 258-263, p.618 - 621, 1998/00
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)no abstracts in English
*; Kunugi, Tomoaki
Microscale Thermophys. Eng., 1(2), p.137 - 142, 1997/00
no abstracts in English
Iwamoto, Akira; Niita, Koji*; Maruyama, Toshiki; Maruyama, Tomoyuki*
JAERI-Conf 96-009, 133 Pages, 1996/05
no abstracts in English
; Chihara, Junzo
Molecular Simulation, 16, p.31 - 46, 1996/00
Times Cited Count:4 Percentile:17.31(Chemistry, Physical)no abstracts in English
; Chihara, Junzo
Physical Review E, 50(2), p.1317 - 1324, 1994/08
Times Cited Count:28 Percentile:74.82(Physics, Fluids & Plasmas)no abstracts in English
JAERI-M 92-181, 126 Pages, 1992/12
no abstracts in English
; G.Kahl*
Europhysics Letters, 18(5), p.421 - 426, 1992/03
Times Cited Count:19 Percentile:72.97(Physics, Multidisciplinary)no abstracts in English
Igawa, Naoki; Ono, Hideo; Nagasaki, Takanori; Ishii, Yoshinobu; Noda, Kenji; Watanabe, H.; Matsuo, Toru*; Igarashi, Kazuo*
Ceramic Transactions, Vol.27, p.135 - 156, 1992/00
no abstracts in English
*;
JAERI-M 85-118, 37 Pages, 1985/08
no abstracts in English
; Furukawa, Kazuo; *; *; *
J.Chem.Soc.,Faraday Trans.,1, 79, p.463 - 471, 1983/00
no abstracts in English